Basic Chemistry
Some basic chemistry about metals
When different types of metals come into contact with each other, and particularly when water is present, chemical reactions take place.
Why? It’s all because of what chemists refer to as ‘The Galvanic Hierarchy’ of metals. In English, when metals that are more resistant to corrosion touch metals that are less resistant, that contact will eat away the metal that is lower down the pecking order of ‘The Galvanic Hierarchy’.
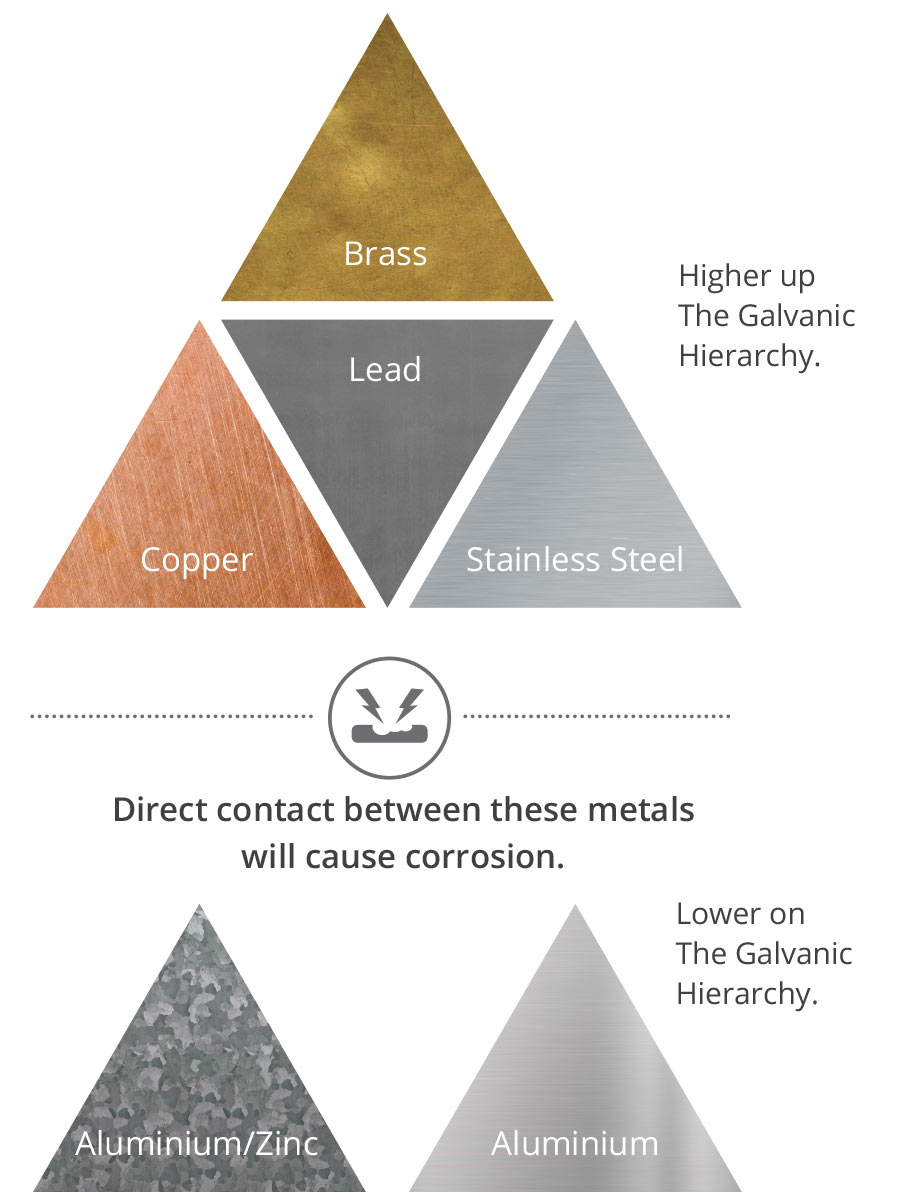
Brass, copper, lead and stainless steel are higher up the galvanic hierarchy than the aluminium/zinc-coated steel and aluminium used as ColorCote pre-painted metal substrates. Direct contact between these dissimilar metals will cause the aluminium alloy systems to quickly corrode.
Water coming from copper and brass pipes or spouting can cause corrosion to pre-painted metal roofing and cladding too.
Isolating different metals
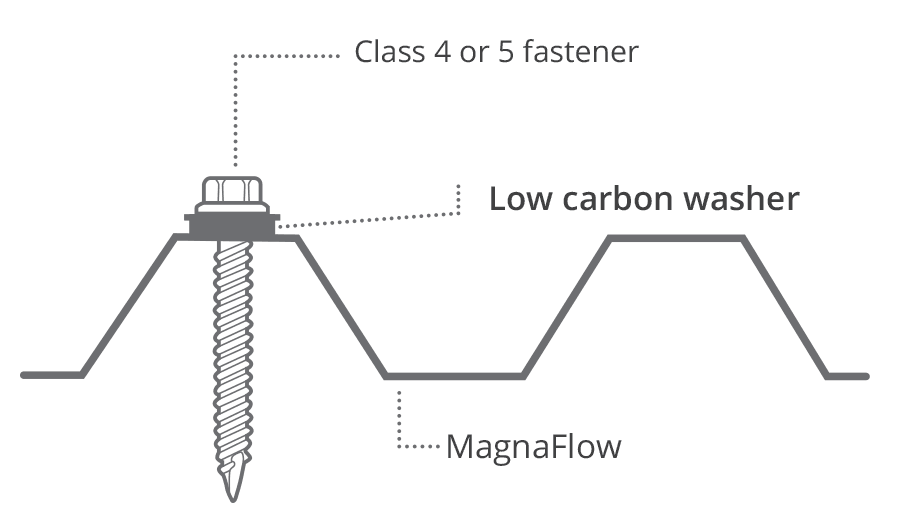
Inert membranes, such as rubber, can be used to isolate different metals. For example, when AlumiGard™ is fixed using stainless steel fasteners, a special low carbon washer is used between these two metals.
Other common building materials cause problems too
Concrete and plaster can cause discolouration to the paint coating which should be protected by an inert membrane where they come into contact.
Tanalised timber and some other timbers such as cedar can cause galvanic corrosion, due to copper used in the treating process. Your roofer should use either an inert membrane at the points of contact (in mild environments only) or fully isolate the timber and roofing materials with a rubber or neoprene gasket.